No Potassium would need to lose one electron and Helium would need to lose two. Together they make the formula LiF.
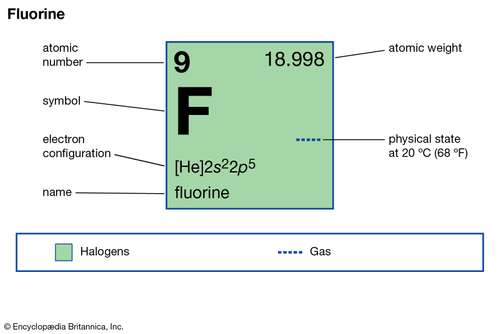
 Fluorine Electron Configuration F With Orbital Diagram
Fluorine Electron Configuration F With Orbital Diagram
One lithium Li atom can combine with one fluorine F atom.
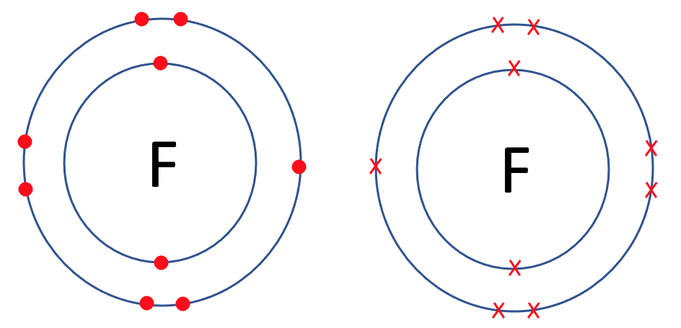
Would fluorine gain one electron. Since the fluorine atom has now seven negative species electrons in its valence shell and thus requires only one electron to completely fill the octet and gains high stability. Consider the example of fluorine see Figure below. A fluorine atom can get a full valence shell by either gaining one more electron or by losing seven electrons.
A fluorine F atom gains one electron to become a fluoride ion. In order to be come stable it would need 1 additional electron. By gaining the electron it will have overall a negative charge.
Is most likely to gain one electron to form an ion with a 1-. If atoms gain electrons they become negative ions or anions. Two fluorine atoms can each sacrifice one of their valence electrons to form a single bond between the atoms.
The transfer of electrons in order to achieve a noble gas configuration is the process known as ionic bonding and this will be covered in more detail later in this chapter. Write the symbol used to represent this ion. So the fluorine atom has eight electrons and a filled outer shell.
This makes the fluorine ion also known as fluoride more stable. Therefore fluorine tend to gain the. However the bond consists of two additional valence electrons for each fluorine atom as the electrons.
Reactivity is an elements ability to gain an electron. The thing that makes fluorine so reactive is its electronegativity. Furthermore does fluorine gain or lose electrons.
Chlorine in its normal state has seven valence electrons. It can lose one of its electrons making it an ion. As fluorine is a halogen the group in which the elements are more reactive as they are one electron lesser than that of the octet configurationand hence it can only gain electrons.
By gaining a negative electron it has an overall negative charge. No Chlorine would need to gain one electron and Bromine would need to gain one electron Are Chlorine and Bromine likely to form an ionic compound. Because fluorine has gained one electron it now has one negative charge.
1 Fluorine will prefer to gain electron rather than loosing electrons. Lithium gives up its one electron to make both atoms happy. Fluorine has seven electrons of its own.
A fluorine atom has nine protons and nine electrons so it is electrically neutral. This is the fluoride anion and it is shown as F -. It now has more positive protons than electrons so it has an overall positive charge.
The former requires the transfer of less electrons so the fluorine atom will try to gain one electron first. Yahoo Answers is shutting down. Therefore F ions are more common than F7 ions.
If a fluorine atom gains an electron it becomes a fluoride ion with an electric charge of -1. A fluorine atom will tend to gain rather than lose an electron. Therefore since fluorine has a higher electronegatvity than chlorine fluorine is more reactive.
Fluorine is in Group VII and a single fluorine atom has seven valence electrons. The fluorine atom has seven electrons in their valence shell and thus tends to gain only one electron to complete the octet in the valence shell. Fluorine in particular does have 7 atoms in its outer shell and by octet8 rule gaining one electron would make 8 electrons.
Now each fluorine atom has six valence electrons to itself. So the better it is at stealing electrons the more reactive it will be. However by the Octet Rule it would like to gain one electron to get a full octet of valence electrons.
Thus fluorine atom tends to gain only one electron. So the valence electrons of fluorine are bound to the atom more strongly than any other atom does. Fluorine compared to other elements is more Electronegative because it requires only one electron to complete an octate and attain stability and the distance at which valence electrons reside is the minimum as compared with other elements having 7 electrons in the outermost orbit.
Atomic Structure The Atom Fluorine Gaining Electrons
 Fluorine Valence Electrons Fluorine Valency F With Dot Diagram
Fluorine Valence Electrons Fluorine Valency F With Dot Diagram

Komentar
Posting Komentar